|
|
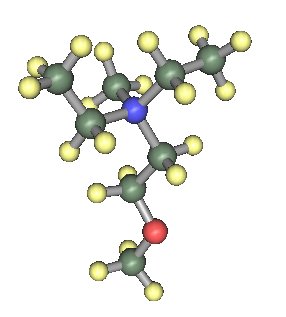
Fig. 1 |
|
|
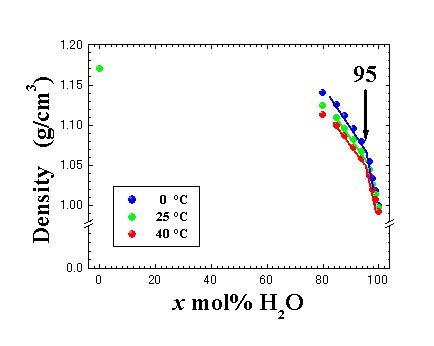
Fig. 2 Temperature and concentration dependences of density in [DEME][BF4]-x mol% H2Omixtures. |
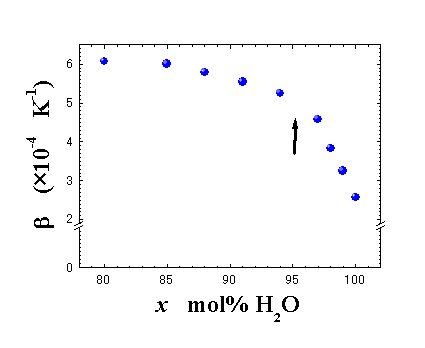 |
Fig. 3. Concentration dependence of volumetric thermal expansion coefficient, À, at 25.C. |
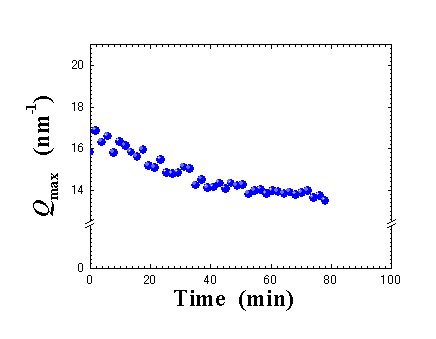 |
Fig. 4. Time dependence of Qmax position. |
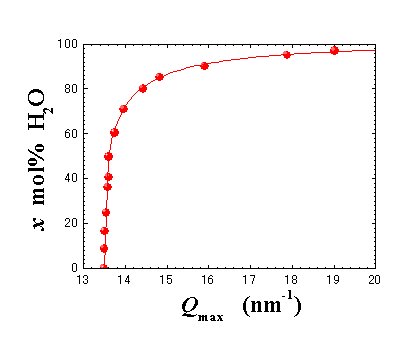 |
Fig. 5. x-Qmax relationship. Solid curve is obtained by the least square fitting method. |
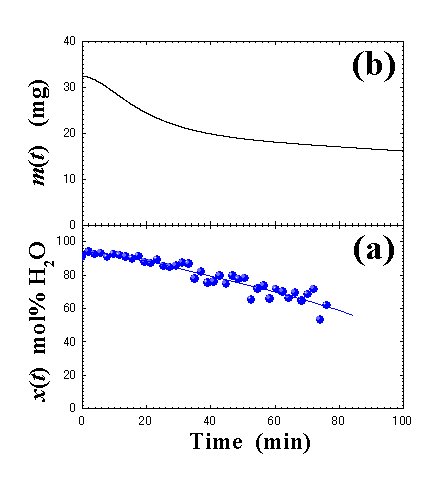 |
Fig. 6. Time dependences of (a) water concentrations and (b) mass. |
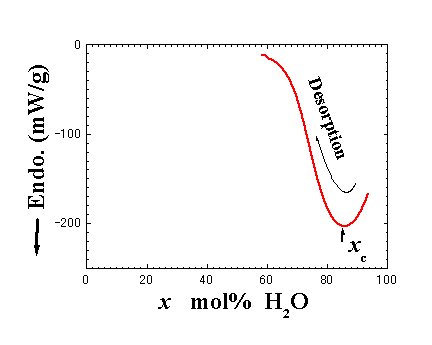 |
Fig. 7. Water concentration dependence of normalized heat flow. |
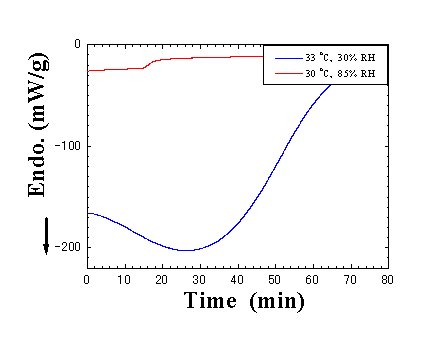 |
Fig. 8. Time dependence of the normalized heat flow. For a comparison, the normalized heat flow at 30.C and 85%RH is plotted as background, where a little mass change was measured after the DSC scan. |
References
[2] H. Katayanagi, K. Nishikawa, H. Shimozaki, K.Miki, P.Westh, and Y. Koga, gMixing schemes in ionic liquid-H2O systems: a thermodynamic study,h Journal of Physical Chemistry B, vol. 108, no. 50, pp. 19451-19457, 2004.
[3] G. GarcL.a-Miaja, J. Troncoso, and L. RomanL., gExcess enthalpy, density, and heat capacity for binary systems of alkylimidazolium-based ionic liquids + water,h Journal of Chemical Thermodynamics, vol. 41, no. 2, pp. 161-166, 2009.
[4] U. DomanL ska, gSolubilities and thermophysical properties of ionic liquids,h Pure and Applied Chemistry, vol. 77, no. 3, pp. 543-557, 2005.
[5] A. Riisager, R. Fehrmann, R. W. Berg, R. Van Hal, and P. Wasserscheid, gThermomorphic phase separation in ionic liquid-organic liquid systems.conductivity and spectroscopic characterization,h Physical Chemistry Chemical Physics, vol. 7, no. 16, pp. 3052-3058, 2005.
[6] U. DomanLska and L. M. CasaLs, gSolubility of phosphonium ionic liquid in alcohols, benzene, and alkylbenzenes,h Journal of Physical Chemistry B, vol. 111, no. 16, pp. 4109-4115, 2007.
[7] T. Sato, G.Masuda, and K. Takagi, gElectrochemical properties of novel ionic liquids for electric double layer capacitor applications,h Electrochimica Acta, vol. 49, no. 21, pp. 3603-3611, 2004.
[8] Y. Imai, H. Abe, T. Goto, Y. Yoshimura, Y. Michishita, and H. Matsumoto, gStructure and thermal property of N, N-diethyl-N-methyl-N-2-methoxyethyl ammonium tetrafluoroborate-H2O mixtures,h Chemical Physics, vol. 352, no. 1-3, pp. 224-230, 2008.
[9] H. Abe, Y. Yoshimura, Y. Imai, T. Goto, and H. Matsumoto, gPhase behavior of room temperature ionic liquid-H2O mixtures: N, N-diethyl-N-methyl-N-2-methoxyethyl ammonium tetrafluoroborate,h Journal of Molecular Liquids, vol. 150, no. 1-3, pp. 16.21, 2009.
[10] M. Aono, Y. Imai, Y. Ogata et al., gAnomalous mixing state in room-temperature ionic liquid-water mixtures: N, Ndiethyl- N-methyl-N-(2-methoxyethyl) ammonium tetrafluoroborate,h Metallurgical and Materials Transactions A, vol. 42, no. 1, pp. 37-40, 2011.
[11] A. Kokorin, Ed., Ionic Liquids: Theory, Properties, New Approaches, InTech, 2011.
[12] H. Abe, Y. Imai, T. Takekiyo, and Y. Yoshimura, gDeuterated water effect in a room temperature ionic liquid: N, N-diethyl-N-methyl-N-2-methoxyethyl ammonium tetrafluoroborate,h Journal of Physical Chemistry B, vol. 114, no. 8, pp. 2834-2839, 2010.
[13] H. Abe, T. Mori, R. Abematsu et al., submitted to Journal of Molecular Liquids. In press.
[14] M. Aono, Y. Imai, H. Abe, H. Matsumoto, and Y. Yoshimura, Thermochimica Acta. In press.
[15] D. A. Allen, R. A. Howe, N. D. Wood, and W. S. Howells, gThe structure ofmolten zinc chloride and potassium chloride mixtures,h Journal of Physics Condensed Matter, vol. 4, no. 6, article 005, pp. 1407-1418, 1992.
[16] M. Salanne, C. Simon, P. Turq, and P. A.Madden, gIntermediate range chemical ordering of cations in simple molten alkali halides,h Journal of Physics Condensed Matter, vol. 20, no. 33, Article ID 332101, 2008.
Last Modified: Nov. 1, 2011