|
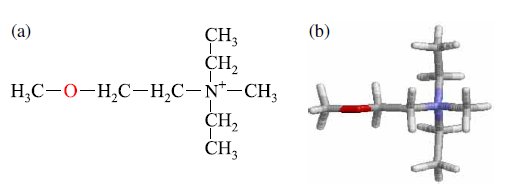
Fig. 1. (a) Chemical and (b) optimized structures of the [DEME] cation are shown. Blue, grey, and red represent the nitrogen, carbon, and oxygen atoms, respectively (color online). |
|
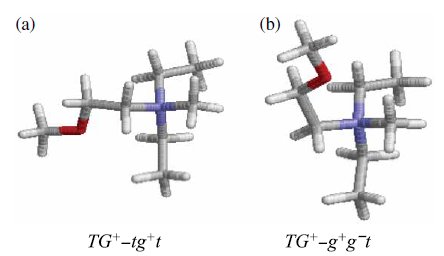 |
Fig. 2. (a) TG+-tg+t and (b) TG+-g+g.t conformers of [DEME] cation are shown. |
|
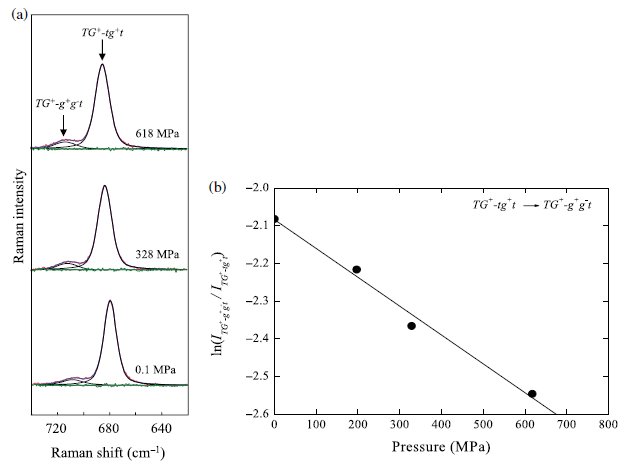
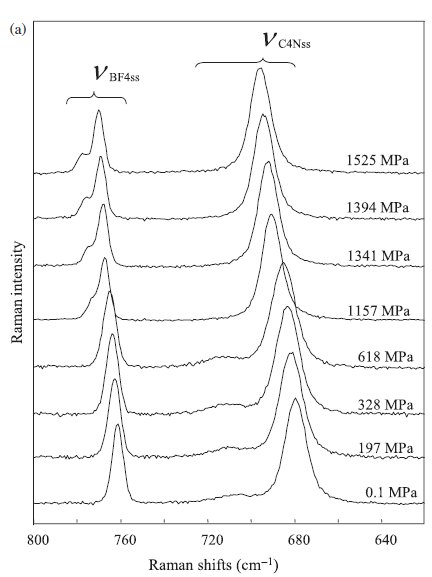
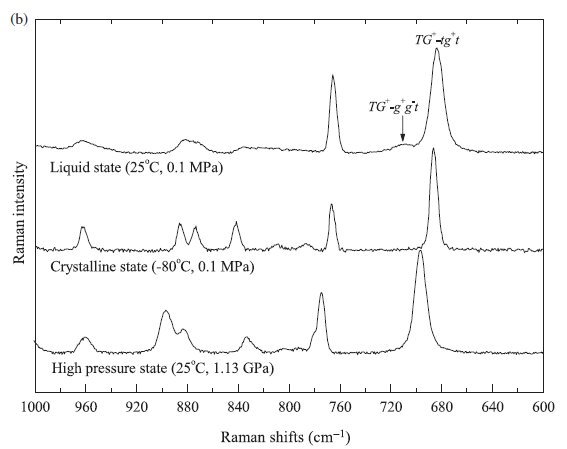
Fig. 3. (a) Raman spectral changes of [DEME][BF4] in the region from 620 to 800 cm-1 as a function of pressure. The peaks at around 680 and 760 cm-1 were assigned to the C4N symmetric stretching mode and BF4- symmetric stretching mode, respectively. In the region of the C4N symmetric stretching mode, the peak at around 680 cm-1 and the weak shoulder at around 710 cm-1 were assigned to the TG+-tg+t and TG+-g+g.t conformers of the [DEME] cation, respectively. (b) The Raman spectra of the liquid state (25 oC, 0.1MPa), the crystalline state (-80 oC, 0.1MPa), and the high pressure state (25 oC, 1.13 GPa). At the crystalline state and the high pressure state, only the TG+-tg+t conformer exists. |
|
|
Fig. 4. (a) Change of integrated Raman intensity of each conformer in the νC4NSS mode as a function of pressure and (b) the pressure dependence of the relative Raman intensity ratio between the two conformers. |
References
[1] T. Sato, G. Matsuda, and K. Takagi, Eletrical properties of novel ionic
liquids for electric double layer capacitor applications, Electrochim. Acta 49 (2004), pp. 3603-3611.
[2] R. Ozawa, S. Hayashi, S. Saha, A. Kobayashi, and H. Hamaguchi, Rotational isomerism and structure of the 1-buthyl-3-methylimidazolium cation in the ionic liquid state, Chem. Lett. 32 (2003), pp. 948-949.
[3] Y. Umebayashi, T. Fujimori, T. Sugizaki, M. Asada, K. Fujii, R. Kanzaki, and S. Ishiguro, Evidence of conformational equilibrium of 1-ethyl-3-methylimidazolium in its ionic liquid salts: Raman spectroscopic study and quantum chemical calculations, J. Phys. Chem. A 109 (2005), pp. 8976-8982.
[4] J.N. Canongia Lopes, K. Shimizu, A.A.H. Padua,Y. Umebayashi, S. Fukuda, K. Fujii, and S. Ishiguro, A tale of two ions: The conformational landscapes of bis (trifluoromethanesulfonyl)amide and N,N-dialkylpyrrolidinium, J. Phys. Chem. B 112 (2008), pp. 1465-1472.
[5] Y. Imai, H. Abe, T. Goto,Y.Yoshimura, S. Kushiyama, and H. Matsumoto, Orientational ordering
of crystal domains in ionic liquid based mixtures, J. Phys. Chem. B 112 (2008), pp. 9841-9846.
[6] Y. Imai, H. Abe, T. Goto, Y. Yoshimura, Y. Michishita, and H. Matsumoto, Structure and
thermal property of N, N-diethyl-N-methyl-N-2-methoxyethyl ammonium tetrafluoroborate-H2O mixtures, Chem. Phys. 352 (2008), pp. 224.230.
[7] Y. Imai, H. Abe, and Y. Yoshimura, X-ray diffraction study of ionic liquid based mixtures,
J. Phys. Chem. B 113 (2009), pp. 2013-2018.
[8] Y. Imai, H. Abe, T. Goto, T. Miyashita, and Y. Yoshimura, Pressure-induced phase transition
of ionic liquid [DEME][BF4]-H2O mixtures, J. Phys. Conf. Ser., in press.
[9] Y. Yoshimura, Y. Goto, H. Abe, and Y. Imai, Existence of nearly-free hydrogen bonds in an ionic liquid, N,Ndiethyl-N-methyl-N-(2-methoxyethyl) ammonium tetrafluoroborate-water at 77 K, J. Phys. Chem. B 113 (2009), pp. 8091-8095.
[10] T. Takekiyo, Y. Imai, H. Abe, and Y. Yoshimura, Raman spectroscopic study and DFT calculations on the
conformational preference of a quaternary ammonium-type ionic liquid, submitted.
[11] H.K. Mao, P.M. Bell, J.W. Shaner, and D.J. Steinberg, Specific volume
measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence
pressure gauge from 0.06 to 1 Mbar, J. Appl. Phys. 49 (1978), pp. 3276-3283.
[12] T. Takekiyo andY.Yoshimura, Raman spectroscopic study on the hydration
structures of tetraethylammonium cation in water, J. Phys. Chem. A 110 (2006), pp. 10829-10833.
[13] T. Takekiyo, M. Kato, andY. Taniguchi, Pressure effect on conformational
equilibria of analog peptides in aqueous solution by Raman spectroscopy,
J. Solution Chem. 33 (2004), pp. 761-775.
National Defense Academy
Last Modified: April 1, 2009