|
|
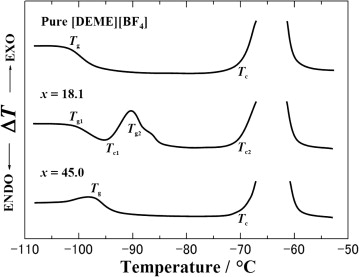
Fig. 1. Schematic DTA warm-up traces of pure [DEME][BF4], 18.1-30.0 mol% H2O mixed solutions are shown. The DTA trace at x = 18.1 gives a first glass transition followed by an exothermic crystallization-like peak at around -95 oC. |
|
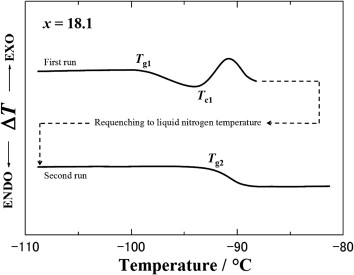 |
Fig. 2. DTA traces with the requenching procedure at x = 18.1 in confirmation of the second glass-transition temperature (Tg2). |
|
|
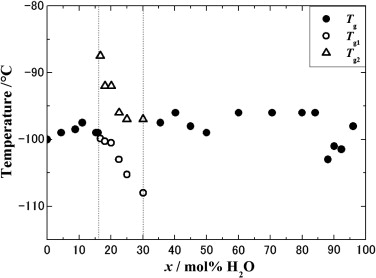
Fig. 3. Tg variations with H2O concentration, x. The dotted vertical lines are guides to the eye. |
References
[2] R. Sheldon, Chem. Commun. 23 (2001) 2399.
[3] T. Sato, G. Masuda, Kentaro Takagi, Electrochim. Acta 49 (2004) 3603.
[4] Y. Jeon et al., J. Phys. Chem. 112 (2008) 923.
[5] H.-C. Chang, J.-C. Jiang, Y.-C. Liou, C.-H. Hung, T.-Y. Lai, S.-H. Lin, J. Chem. Phys. 129 (2008) 044506-1.
[6] W. Jiang, Y. Wang, G.A. Voth, J. Phys. Chem. B 111 (2007) 4812.
[7] C. Schroder, T. Rudas, G. Neumayr, S. Benkner, O. Steinhauser, J. Chem. Phys. 127 (2007) 234503-1.
[8] K. Nishikawa, K. Tozaki, Chem. Phys. Lett. 463 (2008) 369.
[9] G.J. Kabo, A.V. Blokhin, Y.U. Paulechka, A.G. Kabo, M.P. Shymanovich, J. Chem. Eng. Data. 49 (2004) 453.
[10] O. Yamamuro, Y. Minamimoto, Y. Inamura, S. Hayashi, H. Hamaguchi, Chem. Phys. Lett. 423 (2006) 371.
[11] W. Xu, E.I. Cooper, C.A. Angell, J. Phys. Chem. B 107 (2003) 6170.
[12] C.A. Wamser, J. Am. Chem. Soc. 73 (1951) 409.
[13] M. Anbar, S. Guttmann, J. Phys. Chem. 64 (1960) 1896.
[14] Y. Yoshimura, H. Kanno, J. Solut. Chem. 24 (1995) 633.
[15] H. Kanno, K. Shimada, K. Katoh, Chem. Phys. Lett 103 (1983) 219.
[16] C.A. Angell, E.J. Sare, J. Chem. Phys. 49 (1968) 4713.
[17] A. Inaba, O. Andersson, Thermochim. Acta 461 (2007) 44.
[18] M. Beiner, K. Schroter, E. Hempel, S. Reissig, E. Donth, Macromolecule 32 (1999) 6278.
[19] A. Triolo, O. Russina, H.-J. Bleif, E.D. Cola, J. Phys. Chem. B 111 (2007) 4641.
[20] G.S. Fulcher, J. Am. Ceram. Soc. 6 (1925) 339.
[21] Y. Yoshimura, T. Goto, H. Abe, Y. Imai, J. Phys. Chem. B 113 (2009) 8091.
National Defense Academy
Last Modified: April 1, 2009