|
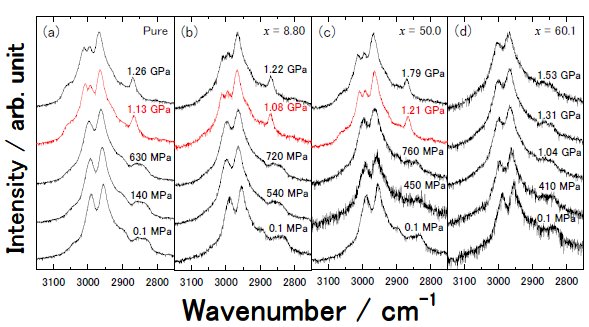 |
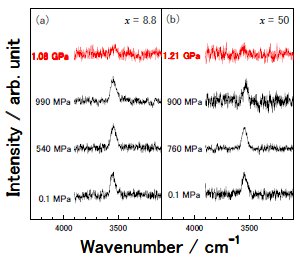
Fig. 1. |
|
|
Fig. 2. ν1 symmetric mode of H2O corresponding to a nearly free hydrogen bond band (NFHB). Upon compression, the NFHB at around 3550 cm-1 seems to fade away. |
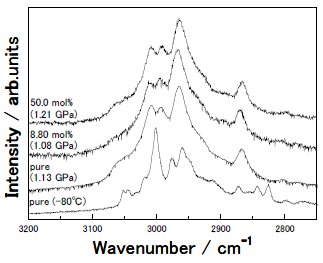 |
Fig. 3. Raman CH stretching spectra at several concentrations where the pressure-induced structural change occurred are shown. For a comparison, the spectrum of pure [DEME][BF4] at -80 °C at a normal pressure is shown at the bottom of the figure. |
References
[2] Jiang W, Wang Y and Voth G A 2007 J. Phys. Chem.B 111, 4812
[3] Schroder C, Rudas T, Neumayr G, Benkner S and Steinhauser O 2007 J. Chem. Phys. 127, 234503
[4] Chang H C, Jiang J C, Liou Y C, Hung C H, Lai T Y and Lin S H 2008 J. Chem. Phys. 129, 044506
[5] Imai Y, Abe H, Goto T, Yoshimura Y, Michishita Y and Matsumoto H 2008 Chem. Phys. 352, 224
[6] Sato T, Masuda G and Takagi K 2004 Electrochim. Act. 49, 3603
[7] Sato T, Masuda G and Takagi K 2008 Electrochim. Act. 53, 4934
[8] Piermarini G J, Block S, Barnett J D and Forman R A 1975 J. Appl. Phys. 46, 2774
[9] Kanno H and Hiraishi J 1981 Chem. Phys. Lett. 83, 452
[10] Imai Y, Abe H and Yoshimura Y 2009 J. Phys. Chem. B 113, 2012
National Defense Academy
Last Modified: April 1, 2009