|
|
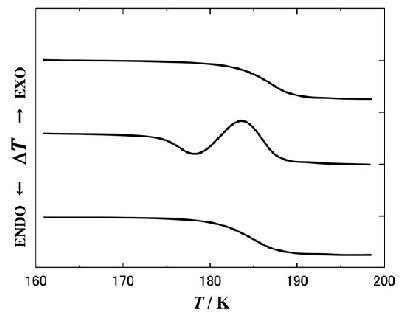
Fig. 1 |
|
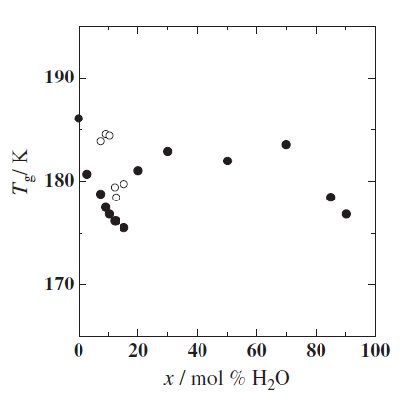 |
Fig. 2 Plot of the of the glass transition temperatures Tg (*) and Tcc (*) as a function of x. |
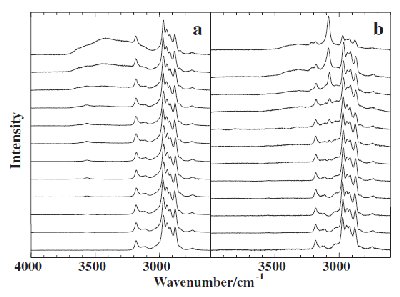 |
Fig. 3. The spectral changes of [bmim][BF4]-H2O solutions probed by the Raman CH and OH stretching spectra in the wavenumber region of (2600 to 4000) cm-1 as a function of x. (a) T = 298 K (room temperature), (b) T = 77 K. The peak heights are normalized by the strongest peak of CH stretching vibration of [bmim][BF4]. (from top to the bottom, x mol% H2O = (95.0, 90.0, 80.0, 70.0, 60.0, 50.0, 40.0, 30.0, 20.0, 10.0, 5.0, and 0.0). |
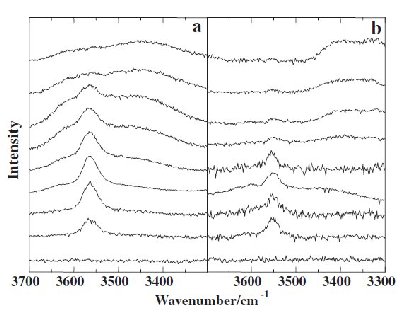 |
Fig. 4. Spectral changes of the nearly-free hydrogen bonded band (NFHB) in the OH stretching spectra ranging between wavenumber (3300 and 3700) cm-1: (a) T = 298 K (room temperature) and (b) T = 77 K. The peak heights are normalized by the strongest peak of CH stretching vibration of [bmim][BF4] (from top to the bottom, x mol% H2O = (95.0, 90.0, 80.0, 70.0, 60.0, 50.0, 30.0, 15.0, 5.0, and 0.0). |
References
[2] T. Welton, Chem. Rev. 99 (1999) 2071-2084.
[3] F. Endres, S.Z.E. Abedin, Phys. Chem. Chem. Phys. 8 (2006) 2101-2116.
[4] T. Sato, G. Masuda, K. Takagi, Electrochim. Acta 49 (2004) 3603-3611.
[5] A. Triolo, O. Russina, H.-J. Blelf, E.D. Cola, J. Phys. Chem. B 111 (2007) 4641-4644.
[6] W. Jiang, Y. Wang, G.A. Voth, J. Phys. Chem. B 111 (2007) 4812-4818.
[7] Y. Imai, H. Abe, T. Miyashita, T. Goto, H. Matsumoto, Y. Yoshimura, Chem. Phys. Lett. 486 (2010) 37-39.
[8] G.J. Kabo, A.V. Blokhin, Y.U. Paulechka, A.G. Kabo, M.P. Shymanovich, J. Chem. Eng. Data 49 (2004) 453-461.
[9] W. Xu, Li-Min Wang, R.A. Nieman, C.A. Angell, J. Phys. Chem. B 107 (2003) 11749-11756.
[10] K. Nishikawa, K. Tozaki, Chem. Phys. Lett. 463 (2008) 369-372.
[11] J.G. Huddleston, A.E. Visser, W.M. Reichert, H.D. Willauser, G.A. Broker, R.D.Rogers, Green Chem. 3 (2001) 156-164.
[12] K. Miki, P. Westh, K. Nishikawa, Y. Koga, J. Phys. Chem. B 109 (2005) 9014-9019.
[13] C.A. Wamser, J. Am. Chem. Soc. 73 (1951) 409-416.
[14] M. Anbar, S. Guttmann, J. Phys. Chem. 64 (1960) 1896-1899.
[15] Y.G. Jeong, W.H. Jo, S.C. Lee, Fibers Polym. 5 (2004) 245-251.
[16] H.N. Ritland, J. Am. Ceram. Soc. 37 (1954) 370-377.
[17] G.S. Fulcher, J. Am. Ceram. Soc. 8 (1925) 339-355.
[18] H. Kanno, K. Shimada, K. Katoh, Chem. Phys. Lett. 103 (1983) 219-221.
[19] L. Cammarata, S.G. Kazarian, P.A. Salter, T. Welton, Phys. Chem. Chem. Phys. 3 (2001) 5192-5200.
[20] Y. Jeon, J. Sung, D. Kim, C. Seo, H. Cheong, Y. Ouchi, R. Ozawa, H. Hamaguchi, J. Phys. Chem. 112 (2008) 923-928.
[21] A. Paul, P. Kumar, A. Samanta, Chem. Phys. Lett. 402 (2005) 375-379.
[22] M. Moreno, F. Castiglione, A. Mele, C. Pasqui, G. Raos, J. Phys. Chem. B 112 (2008) 7826-7836.
[23] J. Bowers, C.P. Butts, P.J. Martin, M.C. Vergara-Gutierrez, R.K. Heenan, Langmuir 18 (2004) 2191-2198.
[24] L.P. Rebelo, V. Najdanovic-Visak, Z.P. Visak, M. Nunes da Ponte, J. Szydlowski, C.A. Cerderirina, J. Troncoso, L. Romani, J.M.S.S. Esperanca, H.J.R. Guedes, H.C. de Sousa, Green Chem. 6 (2004) 369-381.
Last Modified: April 1, 2011