|
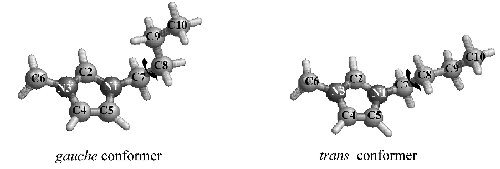 |
Fig. 1 |
|
|
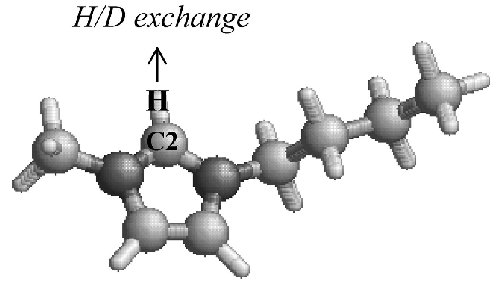
Fig. 2 Schematic representation of the H/D exchange at the C2-H group of the [bmim] cation. |
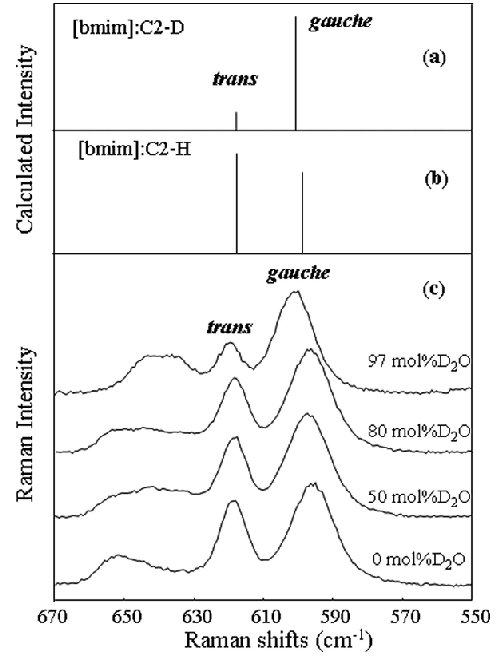 |
Fig. 3. Raman active bands for the gauche and trans conformers with (a) C2-D and (b) C2-H of [bmim] calculated with DFT, respectively. (c) Observed Raman spectral change with varying the D2O concentration x. The peaks at 600 and 620 cm-1 are due from gauche and trans forms of the C7-C8 bond in the butyl chain. The data after 42 days from the sample preparations are displayed. |
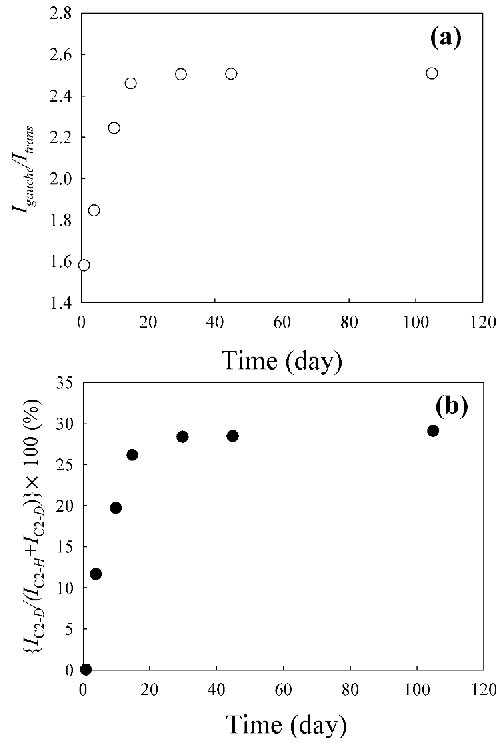 |
Fig. 4. Time evolution of (a) the area ratio (Igauche/Itrans) between the gauche and trans forms and (b) the area ratio {IC2-D/(IC2-H + IC2-D)}[100 (%)] of two peaks for C2-H and C2-D in the combination band arising from the in-plane ring deformation and CH3(N) deformation (νring ip+CH3(N)) vibrations of the D2O mixed solution at x = 40, respectively. |
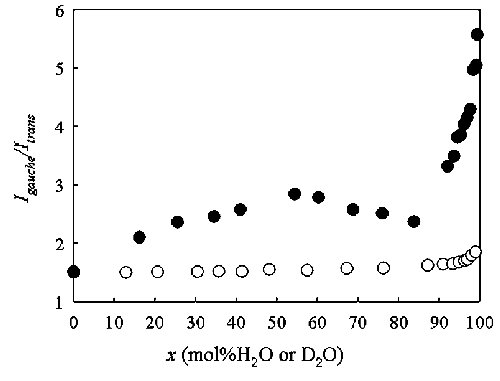 |
Fig. 5. Variations in area ratios (Igauche/Itrans) between the gauche and trans forms of the butyl chain in [bmim][BF4].water mixed solutions as a function of water concentration x. The open circles show the results in the H2O.[bmim][BF4] solutions by Gaussian. Lorentzian fittings for the respective peaks of the gauche and trans forms. The filled circles correspond to the results in the D2O. [bmim][BF4] solutions. |
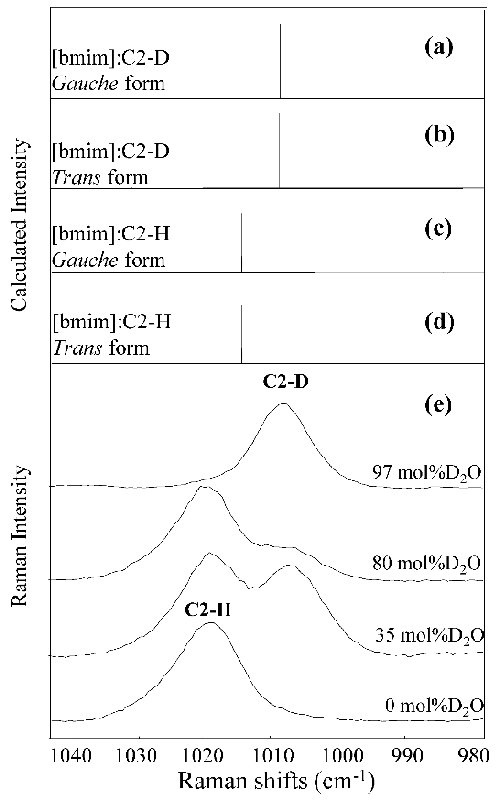 |
Fig. 6. (a-d) Raman active bands for νring ip+CH3(N) vibrations calculated with DFT. (e) Observed Raman spectral change of νring ip+CH3(N) vibrations with varying D2O concentration x. |
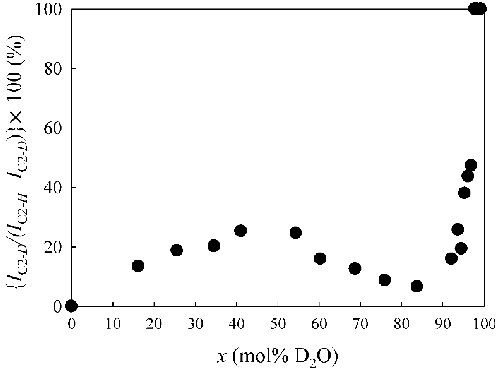 |
Fig. 7. Area ratio {IC2-D/(IC2-H + IC2-D)}[100 (%)] of two peaks for C2-H and C2-D in νring ip+CH3(N) vibrational band against D2O concentration x. The filled circles correspond to the results by Gaussian.Lorentzian fittings for the respective peaks. We show the data after 42 days from the sample preparations. |
References
(2) Cammarata, L.; Kazarian, S. G.; Salter, P. A..; Welton, T. Molecular States of Water in Room Temperature Ionic Liquids. Phys. Chem. Chem. Phys. 2001, 3, 5192-5200.
(3) Saha, S.; Hamaguchi, H. Effect of Water on the Molecular Structure and Arrangement of Nitrile-Functionalized Ionic Liquids. J. Phys. Chem. B 2006, 110, 2777-2781.
(4) Jeon, Y.; Sung, J.; Kim, D.; Seo, C.; Cheong, H.; Ouchi, Y.; Ozawa, R.; Hamaguchi, H. Structural Change of 1-Butyl-3-methylimidazolium Tetrafluoroborate + Water Mixtures Studied by Infrared Vibrational Spectroscopy. J. Phys. Chem. B 2008, 112, 923-928.
(5) Yoshimura, Y.; Goto, T.; Abe, H.; Imai, Y. Existence of Nearly-Free Hydrogen Bonds in an Ionic Liquid, N,N-Diethyl-N-Methyl-N-(2-methoxyethyl) Ammonium Tetrafluoroborate-Water at 77 K. J. Phys. Chem. B 2009, 113, 8091-8095.
(6) Adhikari, A.; Sahu, K.; Dey, S.; Ghosh, S.; Mandal., U.; Bhattacharyya, K. Femtosecond Solvation Dynamics in a Neat Ionic Liquid and Ionic Liquid Microemulsion: Excitation Wavelength Dependence. J. Phys. Chem. B 2007, 111, 12809-12816.
(7) Jiang, W.; Wang, Y.; Voth, G. A. Molecular Dynamics Simulation of Nanostructural Organization in Ionic Liquid/Water Mixtures. J. Phys. Chem. B 2007, 111, 4812-4818.
(8) Moreno, M.; Castiglione, F.; Mele, A.; Pasqui, C.; Raos, G. Interaction of Water with the Model Ionic Liquid [bmim][BF4]: Molecular Dynamics Simulations and Comparison with NMR Data. J. Phys. Chem. B 2008, 112, 7826-7836.
(9) Kato, H.; Miki, K.; Mukai, T.; Nishikawa, K.; Koga, Y. Hydrophobicity/Hydrophilicity of 1-Butyl-2,3-dimethyl and 1-Ethyl-3-methylimidazolium Ions: Toward Characterization of Room Temperature Ionic Liquids. J. Phys. Chem. B 2009, 113, 14754-14760.
(10) Ozawa, R.; Hayashi, S.; Saha, S.; Kobayashi, A.; Hamaguchi, H. Rotational Isomerism and Structure of the 1-Butyl-3-methylimidazolium Cation in the Ionic Liquid State. Chem. Lett. 2003, 32, 948-949.
(11) Berg, R. W.; Deetlefs, M.; Seddon, K. R.; Shim, I.; Thompson, J. M. Raman and ab Initio Studies of Simple and Binary 1-Alkyl-3-methylimidazolium Ionic Liquids. J. Phys. Chem. B 2005, 109, 19018-19025.
(12) Berg, R. W. Raman Spectroscopy and Ab-initio Model Calculations on Ionic Liquids. Monatsh. Chem. 2007, 138, 1045-1075.
(13) Nakakoshi, M.; Ishihara, S.; Utsumi, H.; Seki, H.; Koga, Y.; Nishikawa, K. Anomalous Dynamic Behavior of Ions and Water Molecules in Dilute Aqueous Solution of 1-Butyl-3-methylimidazolium Bromide Studied by NMR. Chem. Phys. Lett. 2006, 427, 87-90.
(14) Yasaka, Y.; Wakai, C.; Matsubayashi, N.; Nakahara, M. Slowdown of H/D Exchange Reaction Rate and Water Dynamics in Ionic Liquids: Deactivation of Solitary Water Solvated by Small Anions in 1-Butyl-3-methylimidazolium Chloride. J. Phys. Chem. A 2007, 111, 541-543.
(15) Ohta, S.; Shimizu, A.; Imai, Y.; Abe, H.; Hatano, N.; Yoshimura, Y. Peculiar Concentration Dependence of H/D Exchange Reaction in 1-Butyl-3-methyl-imidazolium Tetrafluoroborate-D2O Mixtures. Open J. Phys. Chem. 2011, 1, 70-76.
(16) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J. W.; Petersson, G. A.; Ayala, P. W.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavechari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayaakkara, A.; Gonzalez, C.; Challacombe, M. P.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 03; Gaussian, Inc.: Pittsburgh, PA, 2003.
(17) Becke, A. D. Density-Functional Exchange-Energy Approximation with Correct Asmptotic Behavior. Phys. Rev. A 1988, 38, 3098-3100.
(18) Lee, C.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1998, 37, 785.
(19) Huddleston, J. G.; Visser, A. E.; Reichert, W. M.; Willauer, H. D.; Broker, G. A.; Rogers, R. D. Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation. Green Chem. 2001, 3, 156-164.
(20) Tseng, M.; Liang, Y.; Chu, Y. Synthesis of Fused Tetrahydro-β-carbolinequinoxalinones in 1-n-Butyl-2,3-dimethylimidazolium Bis-(trifluoromethylsulfonyl)imide ([bdmim][Tf2N]) and 1-n-Butyl-2,3-dimethylimidazolium Perfluorobutylsulfonate ([bdmim][PFBuSO3]) Ionic Liquids. Tetrahedron Lett. 2005, 46, 6131-6136.
(21) Freire, M. G.; Neves, C. M. S. S.; Marrucho, I. M.; Coutinho, J. A. P.; Fernandes, A. M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744-3749.
(22) Jeon, Y.; Sung, J.; Seo, C.; Lim, H.; Cheong, H.; Kang, M.; Moon, B.; Ouchi, Y.; Kim, D. H. Structures of Ionic Liquids with Different Anions Studied by Infrared Vibration Spectroscopy. J. Phys. Chem. B 2008, 112, 4735-4740.
Last Modified: Nov. 1, 2011