|
|
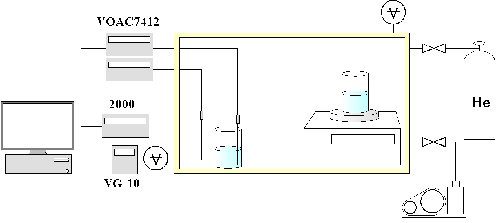
Fig. 1 |
|
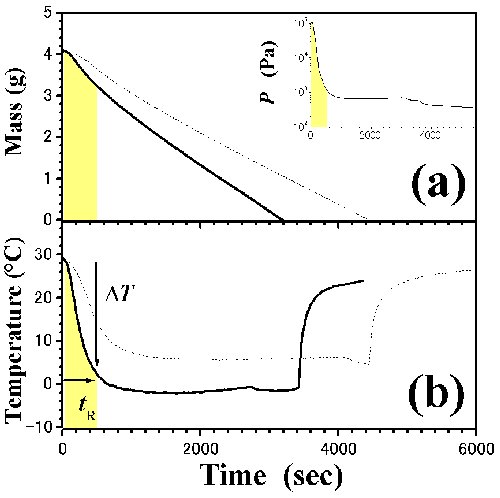
Fig. 2 Time dependences of (a) mass and (b) temperature in pure n-C3H7OH and i-C3H7OH. Dotted and solid lines indicate the n-C3H7OH and i-C3H7OH, respectively. Pressure in i-C3H7OH is expressed in the inset of Fig. 2(a). Each yellow region reveals pressure jump as a non-equilibrium state. Due to the pressure jump, it takes relaxation time, tR, from ambient pressure to low-pressure. |
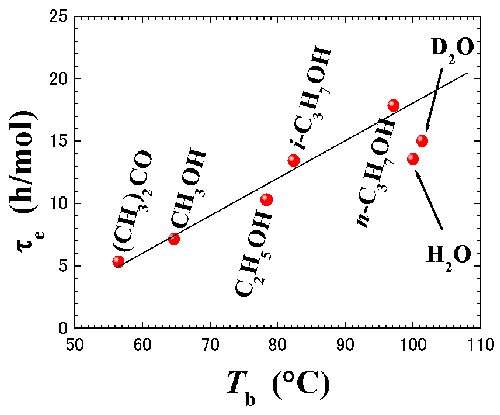 |
Fig. 3. Normalized evaporation time, τe, for pure liquids against the boiling point, Tb. |
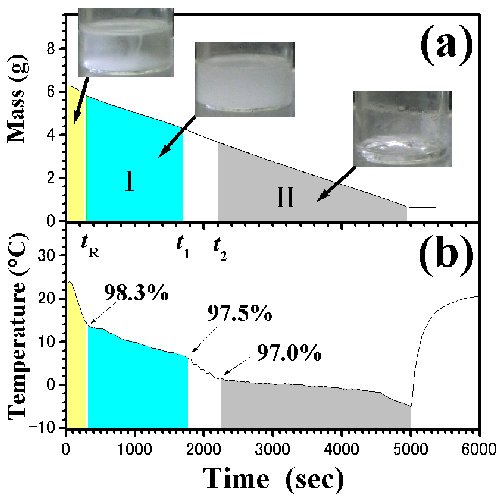 |
Fig. 4. Time dependences of (a) mass and (b) temperature in [DEME][TFSI]-i-C3H7OH. tR is the relaxation time from ambient pressure to low-pressure. Region I (tR<t<t1) and II (t2<t<td) were determined by the visual observations. Pictures by a digital camera are inserted in the figure. The initial concentration, x(t0), is 98.4 mol%. |
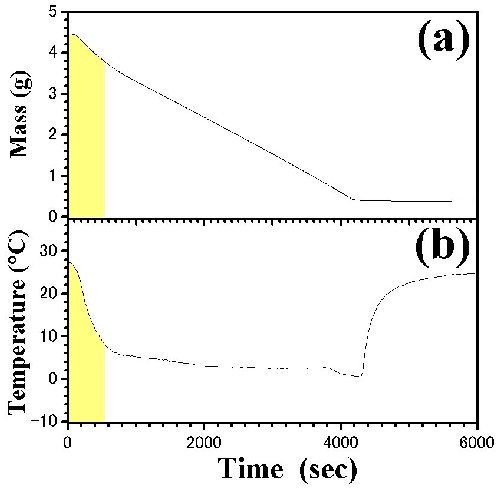 |
Fig. 5. Time dependences of (a) mass and (b) temperature in [DEME][TFSI]-n-C3H7OH. The initial concentration, x(t0), is 98.8 mol%. |
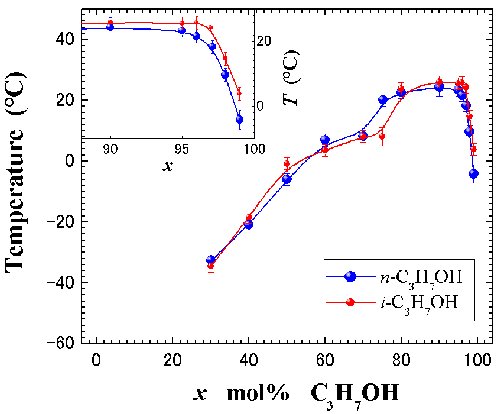 |
Fig. 6. Liquid.liquid phase diagram at ambient pressure. Closed blue and red circles represent [DEME][TFSI]-n-C3H7OH and -i-C3H7OH, respectively. |
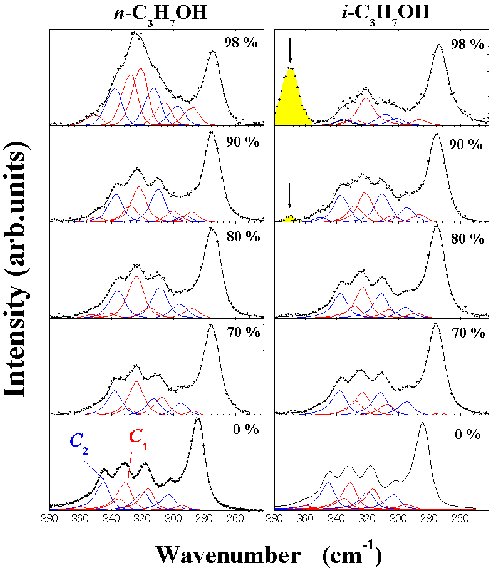 |
Fig. 7. Raman spectra of [DEME][TFSI]-n-C3H7OH and -i-C3H7OH. The red solid lines are contributions from C1 anion conformers. The C2 ones are expressed by the blue solid lines. Arrows in the i-C3H7OH mixtures indicate the bulk i-C3H7OH band. |
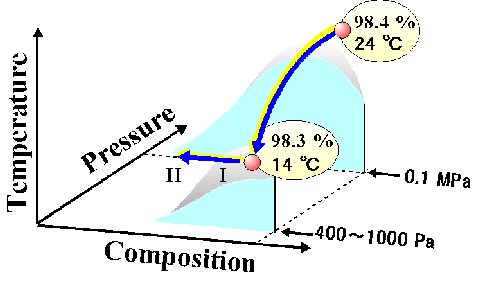 |
Fig. 8. Schematic liquid.liquid equilibrium diagram of [DEME][TFSI]-i-C3H7OH. Gray parts reveal the region of a precursor phenomenon of phase separation. |
References
[2] M.J. Earle, J.M.S.S. Esperanca, M.A. Gilea, J.N.C. Lopes, L.P.N. Rebelo, J.W. Magee, K.R. Seddon, J.A. Widegren, Nature 439 (2006) 831.
[3] O. Russina, A. Triolo, L. Gontrani, R. Caminiti, D. Xiao, L.G. Hines Jr., R.A. Bartsch, E.L. Quitevis, N. Plechkova, K.R. Seddon, Journal of Physics: Condensed Matter 21 (2009) 424121.
[4] Y. Imai, H. Abe, T. Goto, Y. Yoshimura, Y. Michishita, H. Matsumoto, Chemical Physics 352 (2008) 224.
[5] H. Abe, Y. Yoshimura, Y. Imai, T. Goto, H. Matsumoto, Journal of Molecular Liquids 150 (2009) 16.
[6] Y. Imai, H. Abe, Y. Yoshimura, The Journal of Physical Chemistry. B 113 (2009) 2013.
[7] H. Abe, Y. Imai, T. Takekiyo, Y. Yoshimura, The Journal of Physical Chemistry. B 114 (2010) 2834.
[8] M. Aono, Y. Imai, Y. Ogata, H. Abe, T. Goto, Y. Yoshimura, T. Takekiyo, H. Matsumoto, T. Arai, Metallurgical and Materials Transactions 42A (2011) 37.
[9] A. Kokorin (Ed.), Ionic Liquids: Theory, Properties, New Approaches, 2011, (InTech).
[10] J.C. Lassegues, J. Grondin, R. Holomb, P. Johansson, Journal of Raman Spectroscopy 38 (2007) 551.
[11] Y. Yoshimura, T. Takekiyo, Y. Imai, H. Abe, J. Phys. Chem. C (in press).
[12] A. Riisager, R. Fehrmann, R.W. Berg, R. van Hal, P.Wasserscheid, Physical Chemistry Chemical Physics 7 (2005) 3052.
[13] U. Domanska, Pure and Applied Chemistry 77 (2005) 543.
Last Modified: May 1, 2012